
Thermal denaturation of Yfh1 at comparable anion and cation... Download Scientific Diagram
2 Methods The molecular dynamics simulations were performed with the CP2K package. The simulation cell of cubic shape (with a lateral length of 27.6 Å) contained 700 water molecules, one Ca 2+ and one Cl −. The trajectories were gathered in the NVT ensemble, with a timestep of 1 fs.

Cation vs AnionDifference between cation and anionCation and anion differenceCation and Anion
The formula of Calcium Chloride The chemical formula of calcium chloride can be given as CaCl2. It is an ionic compound that consists of one calcium cation Ca2+ and two chloride anions.
Anion Stock Illustrations, Images & Vectors Shutterstock
Exercise 8.11. 1. Write the complete ionic equation for. CaCl 2 ( aq) + Pb ( NO 3) 2 ( aq) → Ca ( NO 3) 2 ( aq) + PbCl 2 ( s) Answer. You may notice that in a complete ionic equation, some ions do not change their chemical form; they stay exactly the same on the reactant and product sides of the equation.

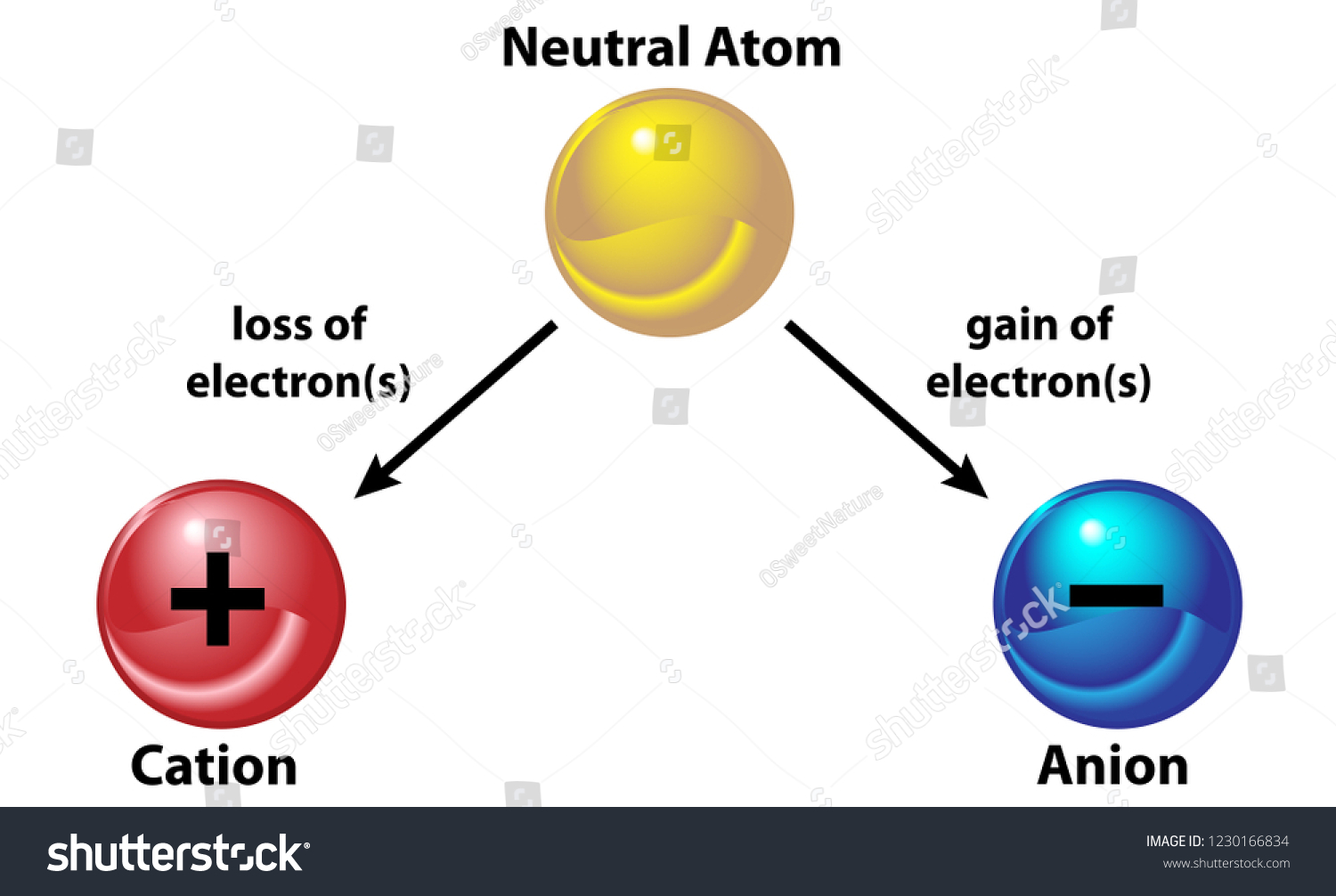
1 Show the formation of cacl2 by the transfer of electrons 2 identify the cation and anion
Solution Verified by Toppr CaCl2 is made up of two ions, The cation is Ca2+ and the anion is Cl− Was this answer helpful? 0 Similar Questions Q 1 Which of the following sets contain only isoelectronic ions? (i) Zn2+,Ca2+,Ga3+,Al3+ (ii) K+,Ca2+,Sc3+,Cl− (iii) P 3−,S2−,Cl−,K+ (iv) T i4+,Ar,Cr3+,v5+ View Solution Q 2
2M of100mL Na2SO4 is mixed with 3M of100mLNaCl solution 1M of200 mL CaCl2 solution then,the
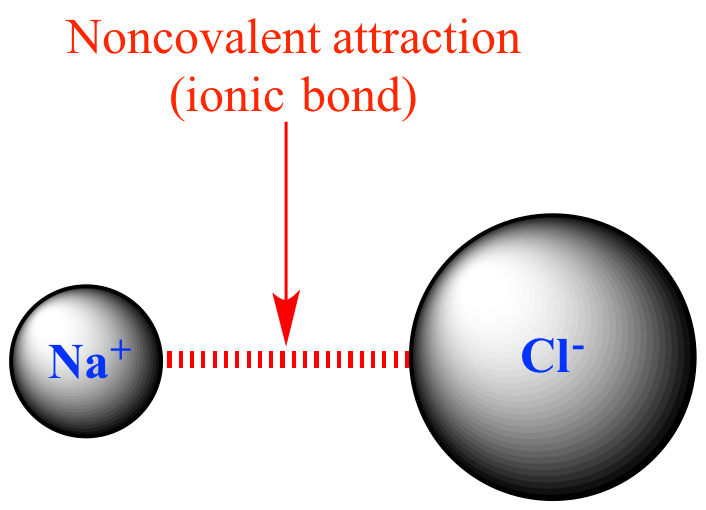
Calcium chloride is a chemical often referred to as a salt. In chemistry, the term salt refers to a compound featuring an ionic bond between a cation and an anion. Ionic bonds form between.

List of Cations and Anions Ion Hydrogen
Precipitation Reactions is shared under a CC BY license and was authored, remixed, and/or curated by LibreTexts. Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. Whether or not such a reaction occurs can be determined by..
SOLVED In the following ionic compound, Calcium chloride (CaCl2), how many electrons must
CaCl2 is an ionic compound owing to the large electronegativity difference between the calcium atom and chlorine atom, which is greater than 2.0. In calcium chloride, the calcium atom donates its two electrons and become cation whereas each chlorine atom gain one electron, donated by Calcium, and get a negative charge.
SOLVED Possible Unknowns CaCO CaCl2 MgSO4 KC1 K2CO3 Na2CO3 NaC2HO2 NH4Cl (NH42SO4 Ca(NO2 KNO3
Common Ion Effect on Solubility. Consider, for example, the effect of adding a soluble salt, such as CaCl 2, to a saturated solution of calcium phosphate [Ca 3 (PO 4) 2 ]. We have seen that the solubility of Ca 3 (PO 4) 2 in water at 25°C is 1.14 × 10 −7 M ( Ksp = 2.07 × 10 −33 ).

2M of 100 ml Na2SO4 is mixed with 3M of 100ml NaCl solution and 1M of 200 ml CaCl2 solution
The ionic formula for Calcium Chloride is CaCl2 Calcium is an Alkaline Earth Metal in the second column of the periodic table. This means that calcium has 2 valence electrons it readily gives away in order to seek the stability of the octet. This makes calcium a Ca+2 cation. Chlorine is a Halogen in the 17th column or p5 group.

Does Calcium (Ca) Form a Cation or Anion? YouTube
A binary ionic compound is a compound composed of a monatomic metal cation and a monatomic nonmetal anion. The metal cation is named first, followed by the nonmetal anion as illustrated in Figure 5.7.1 5.7. 1 for the compound BaCl 2. The word ion is dropped from both parts. Figure 5.7.1 5.7. 1: Naming BaCl2 B a C l 2.

Plot of calcium chlorideextractable P (CaCl2P) against anion storage... Download Scientific
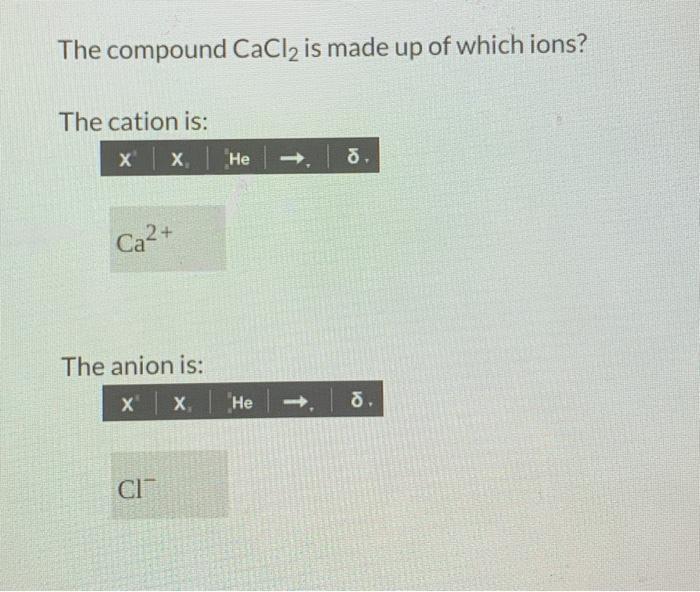
The compound CaCl2 is made up of which ions? The cation is: x x He ю . Ca2+ The anion is: x x He - 8. CI CI" Part 2 (2 points) How many calcium ions are in the compound? 1 How many chloride ions are in the compound? Separate the following balanced chemical equation into its total ionic equation.
Solved The compound CaCl2 is made up of which ions? The
How to make the Chemical Formulas for a merging Cation and Anion? Basically, when a cation like Na (charge +1) and an anion like Cl (charge -1) combine, you get NaCl. . Same thing when Ca (charge +2) and O (charge -2) combine, you get CaO. But when. you combine Ca (charge +2) and Cl (charge -1) you get CaCl2, meaning two atoms of Cl.
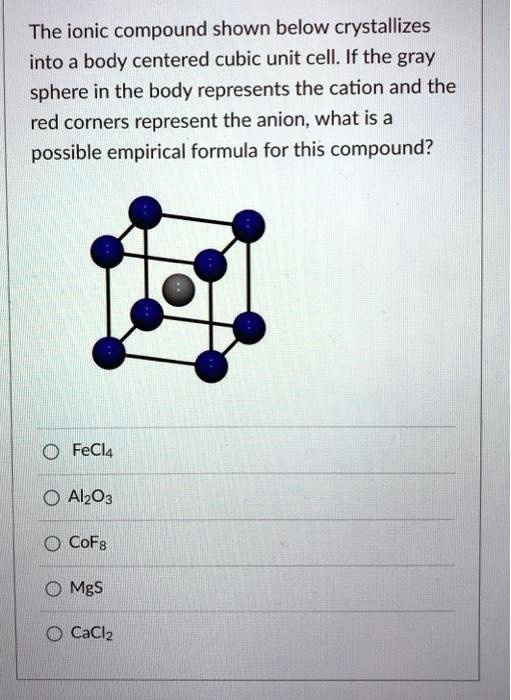
SOLVED The ionic compound shown below crystallizes into a body centered cubic unit cell. If the
Cation vs anion periodic table. It can be possible to predict whether an atom will form a cation or an anion based on its position on the periodic table. Halogens always form anions, alkali metals and alkaline earth metals always form cations. Most other metals form cations (e.g. iron, silver, nickel), whilst most other nonmetals typically form.
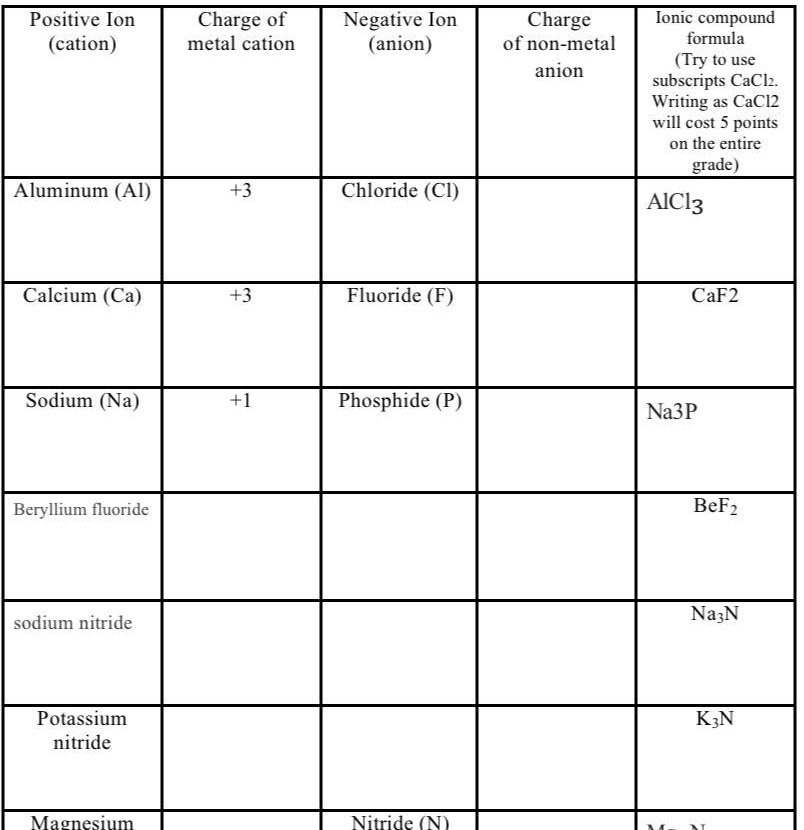
SOLVED 'I need help with this plz ??? Positive Ion (cation) Charge 0f metal cation Negative Ion
Ionic bonding is the complete transfer of valence electron (s) between atoms. It is a type of chemical bond that generates two oppositely charged ions. In ionic bonds, the metal loses electrons to become a positively charged cation, whereas the nonmetal accepts those electrons to become a negatively charged anion.
Illustrated Glossary of Organic Chemistry Anioncation interaction
Chemical Compound Formulas Cacl2 Calcium Chloride - CaCl2 Table of Contents What is Calcium Chloride (CaCl 2 )? Properties of Calcium Chloride - (CaCl 2) Calcium Chloride structure (CaCl 2) Preparation of Calcium Chloride Calcium Chloride Solutions Calcium Chloride (CaCl 2) Uses Health Hazards Frequently Asked Questions

Binary ionic compound practice Name Perio... Physical Chemistry
The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. Most cations have charges of [+1] through [+6] while most ions have charges of [-1] through [-3]. Trick: Set # of Anions = Charge of Cation and. set # of Cations = Charge of Anion.